Abstract
Background: Chimeric antigen receptor T cell (CAR-T) therapies for multiple myeloma (MM) targeting B cell maturation antigen (BCMA) have demonstrated promising efficacy; however, many patients with relapsed/refractory (r/r) MM will ultimately relapse. To improve on existing BCMA CAR-Ts, two critical attributes of the CAR-T product were investigated: 1) the potency of the BCMA CAR construct, and 2) a rapid manufacturing process that would both preserve the stemness of T cells to ensure longer duration of response and provide timely access for patients with rapidly progressing, aggressive disease. Through extensive panning and discriminating CAR-T functional assays to assess performance, we have identified a superior anti-BCMA CAR construct that when combined with an innovative T-Charge™ manufacturing platform, produces a highly potent product.
Methods and Results: We have developed a novel anti-BCMA CAR-T product for the treatment of MM. Our anti-BCMA CAR consists of an extracellular single chain variable fragment (scFv) targeting BCMA, fused to the CD8α hinge and transmembrane domains, followed by CD137 (4-1BB) co-stimulatory and CD3ζ chain signaling domains. Selection of our development candidate was based on a screening of seventeen anti-BCMA CARs, each comprising a distinct scFv derived from phage display libraries and hybridoma with a wide range of affinities and epitopes. One candidate clone from the fully human B cell library was identified after rigorous assessment of transduction efficiency, CAR expression, antigen specificity/selectivity and CAR-T cell function against a MM cell line, both in vitro and in vivo. This scFv also demonstrates high specificity to human BCMA by Retrogenix platform using a commercial human plasma membrane protein array assay. CAR expression and functionality of the CAR constructs were compared in the CAR-T cells generated with two different cell processes: a traditional manufacturing (TM) in which CAR-T cells are expanded in vitro for 9-10 days and a novel T-Charge TM process, which is an expansionless CAR-T manufacturing process that takes <2days to generate functional CAR-Ts.
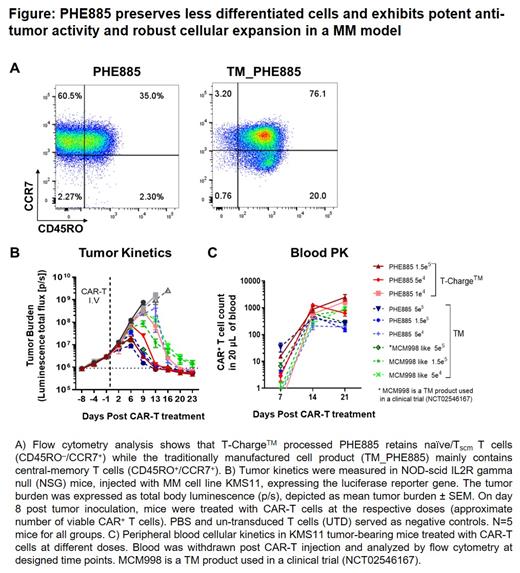
PHE885 generated with the novel T-Charge TM manufacturing platform, retains naïve/ stem cell memory T cell (T scm) (CD45RO -/CCR7 +) while the traditionally manufactured cell product carrying the same scFv (TM_PHE885) mainly contains central-memory T cells (CD45RO +/CCR7 +). In addition to its unique phenotype, PHE885 secretes up to 25 fold more target specific IL-2 and ~7 fold more IFN gamma in vitro, when comparing to the TM products either using the same lentiviral vector (TM_PHE885) or a clinically validated anti-BCMA vector (MCM998). In an immunodeficient NOD-scid IL2R gamma null (NSG) mouse model of MM, PHE885 induced tumor regression at a very low dose in a dose-dependent manner, and was up to 5 fold more efficacious in eradicating tumors compared to the two TM products. This enhanced efficacy was accompanied by higher cellular expansion in vivo (up to 3 fold higher C max and AUC 0-21d). The ability of PHE885 to control the tumor at lower doses with a greater expansion profile confirms the robustness and potency of the PHE885 cells. Furthermore, unlike the TM products, PHE885 induced earlier graft-versus-host disease at medium and high tested doses in this mouse model, suggesting the stemness of the product is most likely the driver of stronger T cell expansion.
Conclusions: PHE885 was discovered and designed to enhance potency and drive persistence of CAR-T through the combination of a novel CAR construct carrying a fully human anti-BCMA scFv fused to 4-1BB/CD3ζ signaling domains and a novel T-Charge TM manufacturing platform, which enables rapid and reliable patient access. The novel T-Charge TM platform allows PHE885 to preserve a significantly higher proportion of naïve/ T scm cells, enabling PHE885 to effectively engraft, expand and reject tumors at a dose of 5 fold lower than TM CAR-T cells. Based on these results, a Phase 1, open-label trial assessing PHE885 in patients with r/r MM (NCT04318327) was initiated. Initial data from the dose escalation portion of the Phase 1 study will be presented separately.
Bu: Novartis: Current Employment, Patents & Royalties: Co-inventor on patent applications. Bennett: BMS: Current Employment; Novartis: Ended employment in the past 24 months, Other: PB was a full-time employee of Novartis at the time of the study. Barton: BMS: Current Employment; Novartis: Other: NB was a full-time employee of Novartis at the time of the study. Bradshaw: Novartis: Other: LB was a full-time employee of Novartis at the time of the study; AstraZeneca: Current Employment. Pinon-Ortiz: NIBR: Current Employment, Current holder of stock options in a privately-held company. Li: Novartis: Current Employment. Vaidya: NIBR: Current Employment. Zhu: Novartis: Current equity holder in publicly-traded company; NIBR: Current Employment. Mansfield: Novartis: Current Employment. Granda: Novartis: Current Employment, Patents & Royalties: No royalties as company-held patents. Guimaraes: Novartis: Current Employment, Patents & Royalties: Co-inventor on patent applications. Treanor: Novartis: Current Employment, Current holder of individual stocks in a privately-held company, Divested equity in a private or publicly-traded company in the past 24 months, Patents & Royalties: no royalties as company-held patents. Brogdon: Novartis Institutes for Biomedical Research: Current Employment.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal